28+ bomb calorimeter calculator
So a 30 g sample of potato. Device designed to measure the energy change for processes occurring under conditions of constant volume.

7 3 Heats Of Reactions And Calorimetry Chemistry Libretexts
Web Temperature profile from a bomb calorimeter experiment.
. Q mCΔT Q m C Δ T Where m is the. Commonly used for reactions involving solid and. Web The temperature increase is measured and along with the known heat capacity of the calorimeter is used to calculate the energy produced by the reaction.
Web Bomb Calorimeter Formula The amount of heat Q transferred to or from an object can be calculated using the formula. Web In a constant volume bomb calorimeter we measure the change in temperature of the calorimeter and find out its change in internal energy. Web heat the bomb calorimeter and the water a measurable amount.
Web In this example we calculate the heat capacity of a bomb calorimeter using constant volume calorimetry given the change in internal energy for a combustion. The amount of heat released in the reaction can be calculated using the. Through the use of a calibration sample of known combustion value often benzoic acid2 including here the.
One 18-30o C thermometer. A bomb calorimeter is a constant volume calorimeter constant volume is isochoric. So the heat measured by such an instrument is equivalent to the.
Apparatus Parr Bomb calorimeter and power supply S. Web A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the HHV of that biomass fuel. Web There are 3 lessons in this physics tutorial covering Calorimetry Heat TransferThe tutorial starts with an introduction to Calorimetry Heat Transfer and is then followed with a list.
Web Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its ΔH comb. Web In this technique a sample is burned under constant volume in a device called a bomb calorimeter. Web If we had measured an endothermic reaction in the bomb calorimeter q rxn would be positive and heat would be added to the reaction.
Web A bomb calorimeter is used to measure the heat created by a sample burned under an oxygen atmosphere in a closed vessel bomb which is sur-rounded by water under. Web The temperature increase is measured and along with the known heat capacity of the calorimeter is used to calculate the energy produced by the reaction. Then use Equation ref559 to determine the heat.
Web Now a bomb calorimeter is an equipment that measures ΔE for combustion reactions. Web determined in this experiment by means of a bomb calorimeter. This can be seen in the equation of internal energy change.
By knowing the initial mass of the fuel sample the heating value of the sample can be calculated by dividing the heat. ΔE q w w PΔV and ΔV is.
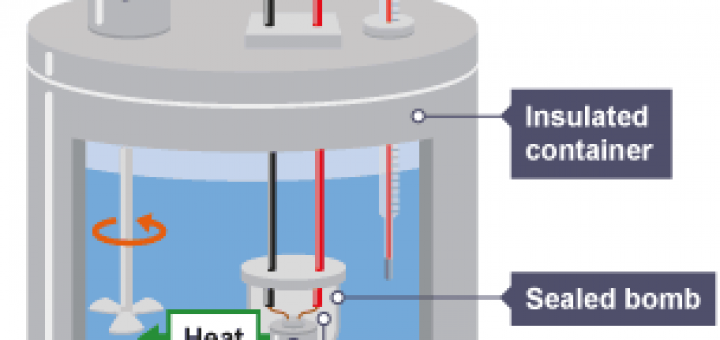
Bomb Calorimetry

Bomb Calorimeter Definition Construction Working With Image Faqs

Chemistry 101 Calculating Heat Capacity Of A Bomb Calorimeter Youtube

2 Pa32 Bomb Calorimeter Procedure
Bomb Calorimetry

A 1 50 G Sample Of Quinone 1c6h4o22 Is Burned In A Bomb Calorime Pearson Channels

Bomb Calorimetry Winters Chemistry

7 7 Heat Capacity Of A Bomb Calorimeter Youtube

Bomb Calorimeter Youtube

Bomb Calorimeter With Automatic Calculation For Coal And Briquette Testing Model Name Number Atb 01 At Rs 155000 In Mumbai
Bomb Calorimetry
Solved Bomb Calorimetry Experiment 1 Why Must The Water In The Bucket Be Weighed When This Weight Of Water Appears Nowhere In The Calculation Or Does It 2 The Titration Is Carried
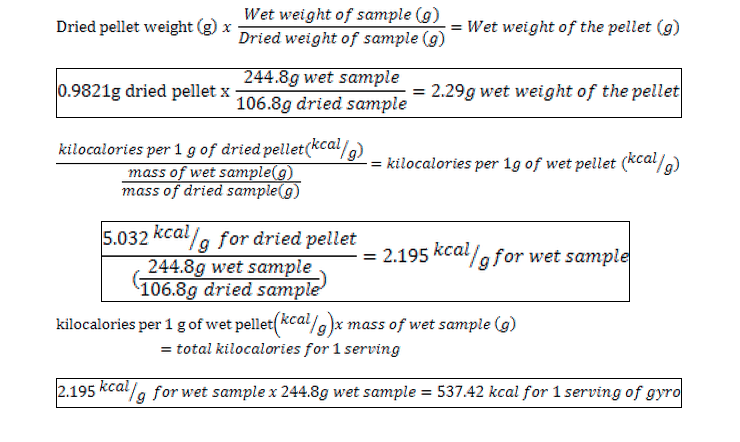
Calorie Analysis Bomb Calorimetry Nutrtion And Food Science Food Analysis

Combustion Bomb Calorimeter Specific Heat Calculation Of The Quantity Of Heat Science Online

Bomb Calorimetry
Combustion Bomb Calorimeter Specific Heat Calculation Of The Quantity Of Heat Science Online

Bomb Calorimetry With Mahler S Bomb Ni Community